
CE Marking
CE stand for: ‘CONFORMITE EUROPENE’ =European Conformance
Mandatory & Regulatory Product Safety Requirement for Exports to the European Union (EU)
What is CE Marking ?
- European Union (EU) Mandatory & Regulatory Requirement addressing product safety
- Mandatory design review and risk identification and mitigation process.
- Minimizes product risks to humans, animals, the environment.
- Manufacturers Self Declaration
- States compliance to the EU Directives
- Guarantees free movement of goods to and within the EU
Elements of CE Marking:
- Directives
- Standards
- Essential Health and Safety requirements
- Compliance Procedures (Conformity Assessment)
- Technical Construction File (TCF)
- EC Declaration of Conformity
- CE Marking
What are EU Directives ?
- Statements of law
- Drafted by the EU Commission and issued by EU council of Ministers
- Spell out Essential Health & Safety Requirements of the product and the User
- Directives explain how manufacturers are able to demonstrate conformity with the essential requirements
- State Legal Objectives to be met
- Have a Legal Standing
- As the directive is a New Approach directive, its text does not provide any technical solutions or details of how its requirements must be fulfilled.
Types of Directives:
- General (General Product Safety, CE Marking etc)
- Generic/Umbrella (Low Voltage Directive, Electromagnetic Compatibility etc.)
- Product Specific (Machinery, Medical Devices, Pressure Equipment, Toys etc.)

CE Marking Directives:
- Simple Pressure Vessels Directive – 2014/29/EU
- Electromagnetic Compatibility Directive – 2014/30/EU
- Non-automatic Weighing Instruments Directive – 2014/31/EU
- Measuring Instruments Directive – 2014/32/EU
- Lifts Safety Directive – 2014/33/EU
- ATEX Directive – 2014/34/EU
- Low Voltage Directive – 2014/35/EU
- Toy Safety Directive – 2009/48/EU
- Restriction of Hazardous Substances in Electrical and Electronic Equipment – 2011/65/EU
- Construction Products Regulation (CPR) – 305/2011/EU
- Radio Equipment Directive – 2014/53/EU
- Pressure Equipment Directive – 2014/68/EU
- Gas Appliances- Regulation (EU) 2016/426
- Cableway Installations – Regulation (EU) 2016/424
- Personal Protective Equipment – Regulation (EU) 2016/425
Elements of Directives:
- Scope
- Essential Requirements (Health, Safety, Environment etc)
- Conformity Assessment Procedures
- Technical File
- CE Marking
What are harmonized Standards ?
A harmonized standard is a technical specification (European standard or harmonization document) adopted by the ‘European Committee for Standardization’ (CEN) and the European Committee for Electro technical Standardization’ (Cenelec).
Harmonized Standards:
- Agreed to by all the members of EU
- Designated “EN” or European Norm.
- Published in the Official Journal (OJ)* of the EU.
- Generally are based on IEC, CEN, CENELEC, CISPR ,ISO or a EU national standards.
- Generally fulfills the requirements of the Applicable European Directives & provide detailed technical information enabling manufacturers to meet the essential requirements
- Identify the technical means (requirements) to meet these legal objectives
Harmonised EN Standards are tool which can be used by:
- Manufacturers, for design, manufacture, inspection, conformity assessment
- Notified Bodies, for carrying out Conformity Assessment procedures
- Market Surveillance authorities, for carrying out inspections and controls
- All safety standards prescribe inspection and testing to ensure compliance with all detail requirements.

Examples of Harmonized Standards:
- EN 415 : Safety of packaging machines
- EN 16092-2 : Machine tools safety – Presses
- EN 14122 : Safety of machinery – Permanent means of access to machinery
- EN 60065 : Audio, video and similar electronic apparatus – Safety requirements
- EN 61010 :Safety requirements for electrical equipment for measurement, control, and laboratory use
- EN 60335 : Household and similar electrical appliances – Safety
- EN 12100 : Safety of machinery – General principles for design – Risk assessment and risk reduction
- EN 60204 : Safety of machinery – Electrical equipment of machines
- EN 60529 : Degrees of protection provided by enclosures (IP Code)
- EN 4413 : Hydraulic fluid power
- EN 4414 : Pneumatic fluid power
- EN 60034 : Rotating electrical machines
- EN 61000 : Electromagnetic compatibility
Role of Standards:
Compliance with the standards means “PRESUMPTION OF CONFORMITY” to the essential health and safety requirements of the all applicable Directives.
Essential Health & Safety Requirements (EHSR):
The necessary requirements to achieve the objective of the relevant directive.Essential Requirements arise from certain risks, hazards and/or environmental concerns associated with certain products and/or product attributes.All Directives identify Essential Health and Safety requirements for products coming under the relevant Directive Essential health and safety requirements are at the heart of the New Approach Directives Compliance with the EHSRs of all the applicable Directives is a must.
CONFORMITY ASSESSMENT:
- Conformity assessment is defined as: ” any activity concerned with determining directly or indirectly that relevant requirements are fulfilled.” Conformity assessment procedures provide a means of ensuring that the products, services, or systems produced or operated have the required characteristics and that these characteristics are consistent from product to product, service to service, or system to system.
- Conformity assessment includes: sampling and testing; inspection; certification; management system assessment and registration; accreditation of the competence of those activities and recognition of an accreditation program’s capability. A specific conformity assessment process may include one or more of these conformity assessment activities. While each of these activities is a distinct operation, they are closely interrelated. In addition, standards are interwoven into all aspects of these activities and can have a major impact on the outcome of a conformity assessment process.
- Conformity assessment activities form a vital link between standards (which define necessary characteristics or requirements for products) and the products themselves. Together standards and conformity assessment activities impact almost every aspect of life in Europe.
CONFORMITY ASSESSMENT May involve one or all of the following:
- Risk Assessment
- Type Tests
- Routine Tests
- Internal Control of Production
- Periodic Audits of the manufacturing location by a Notified Body

Technical File
- Documentary Evidence and Record of CE Marking Process
- Technical files are your documented evidence to show that products properly comply with the requirements of the directives which apply to it.
- Contents laid down in every directive
- Must be “controlled” document
- Must contain all the required information
- Can be a paper file ( Hard Copy) or in Soft format (with back-up)
- One should be able to present it at short notice
- Must be easy to maintain and must be kept up to date
Technical File Contents:
- Part 1: Manufacturer’s profile, Product Description, Intended use
- Part 2: Design & manufacturing drawings
- Part 3: Design documents / Calculations
- Part 4: List of applicable Directives and Standards
- Part 5: Test Reports, Compliance documents
- Part 6: Information for Use ( Manuals)
- Part 7: Quality System Information
- Part 8: List of Safety Critical Components; Product Photographs
- Part 9: Copy of Declaration of Conformity

EC Declaration of Conformity
- A formal statement that products comply with applicable directive and standard
- Signed by responsible person within the organization (e.g. company director)
- It is not evidence of compliance in itself
- Minimum legal requirement
Content of EC Declaration of Conformity :
- Name & Address of the Manufacturer
- Description of the Product /Model/s etc
- List of Applicable Standards and Directives
- Year in which the CE mark is affixed
- Statement to the effect that the product complies to the applicable Directives and Standards
- Signature of the Authorized Signatory of the Manufacturer inc. his/her identification ( Name , Designation ) with the date
- Details of Authorized European Representative
Principles of CE Marking:
- The CE marking symbolizes the conformity of the product with all the applicable Community requirements imposed on the manufacturer.
- The CE marking affixed to products is a declaration by the person responsible that the product conforms to all applicable Community provisions and the appropriate conformity assessment procedures have been completed.
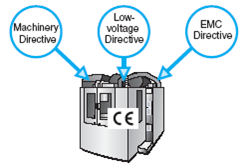
Products to be CE Marked:
- The CE marking is mandatory and must be affixed on a product before it is used in the European Union
- When a product is covered under several CE marking directives, the CE mark indicates that the products conform to the provisions & requirements of all the applicable directives.
- A product may not be CE marked, unless it is covered by a CE MARKING DIRECTIVE.
CE Mark:
The CE conformity marking shall consist of the initials ‘CE’ taking the following form:

If the CE marking is reduced or enlarged the proportions given in the above graduated drawing must be respected.The various components of the CE marking must have substantially the same vertical dimension, which may not be less than 5 mm.
Affixing of the CE Mark:
- The CE marking must be affixed by the manufacturer, or by the authorized representative established within the Community.
- The CE marking must take the proper form. If the CE marking is reduced or enlarged the proportions must be respected.
- The CE marking must be affixed visibly, legibly and indelibly to the product or to its data plate. However, where this is not possible or not warranted on account of the nature of the product, it must be affixed to the packaging, if any, and to the accompanying documents, where the directive concerned provides for such documents.
- Where a notified body is involved in the production control phase according to the applicable directives, its identification number must follow the CE marking. The manufacturer or the authorized representative established in the Community affixes the identification number, under the responsibility of the notified body.

CE: Risks of Non-Compliance
- Market risk: can be shut out of EU markets : all member countries simultaneously ( Blacklisted)
- Financial risk: heavy fines and / or specific liability if an accident occurs
- Criminal sanctions for the person who signs the DoC including imprisonment
BENEFITS OF CE MARKING:
- European Market Growth Potential
- It is Easiest way to export to Europe:Market access to all 32 European countries under one uniform set of product requirements. Additional European countries are expected to mandate CE marking in the near future.
- Customer Safety & Lower Product Liability Risks:Products complying with CE marking requirements may enjoy lower product liability judgments in the case of an accident or injury.
- Enhanced Product Design:Going through the steps in the CE marking will result in a thorough review of your product design and manufacturing process. These steps may bring about a new innovative, and/or cost effective design.
- Improved Production:Some of the conformity assessment modules require the existence of quality assurance programs. These systems can simplify procedures, lower costs, and improve product quality.
- Creation of product / brand image:Besides acceptance in the European Union, ‘CE’ marked Products are preferred in most of the African, Middle East and South East Asian Countries.
- CE marked products always have an edge over those without a CE mark.
- European companies having manufacturing bases in India / tie ups with India companies ,prefer CE Marked manufacturing equipment at their Indian Manufacturing locations.
- Indian Govt. tenders for bulk supply of certain category of Medical devices, IT products, Machinery, asks for a product compliant to the European Safety Standards.

Steps to CE Marking:
- Identify Applicable Directives
- Identify Applicable Standards
- Design the Product to meet the EHSRs of all applicable directives & requirements in the relevant standards
- Identify and ensure all Safety Critical Components are CE Marked
- Carry out Tests
- Compile the Technical File
- Prepare a Declaration
- Affix CE mark on the product

The CE marking is like an ice berg. Over the sea we can find CE marking, Declaration of Conformity, user manuals, etc.
Below the sea all other more complicated and extensive procedures (they need more knowledge, time, resources,..) are hidden: risk assessment, conformity assessment procedures, technical file, special conditions of product use, responsibilities, ….
Prazamana – Your CE Marking Consultant In Pune & India
- As An Authorized Consultant, Prazamana Takes Complete Responsibility For The Declaration Of Conformity
- Identify The Relevant Standards And Directives And Apply It To Your Machinery
- Assess And Report The Risk
- Create A Safety Concept And Machine Safety Design
- Make Necessary Recommendations As Per The Machinery Health And Safety Requirements
- Perform Necessary Checks, Tests And Measurements
- Create Legal Documents
- Manage Certification
We are experts in CE marking implementation and ensure that your machinery receives guaranteed access to the European market. With us, you protect your workforce, enhance productivity and save a lot of your time and money.

Leave a Reply